Formins are a family of proteins conserved across a wide range of eukaryotes and constitute a major class of actin nucleators. In a paper recently published in Molecular Biology of the Cell, a team led by Ph.D. student Brian Graziano in the laboratory of Professor Bruce Goode made the surprising finding that formins depend on co-factors to efficiently nucleate actin assembly both in vitro and in vivo. This discovery was unanticipated because earlier studies had shown that purified formins are sufficient to catalyze actin polymerization in vitro. Graziano, working in collaboration with the labs of Laurent Blanchoin and Isabelle Sagot, investigated the mechanism and function of a formin-binding protein called Bud6 and found that it elevates formin nucleation activity by 5-10 fold. Further, they showed that this activity of Bud6 is critical in vivo for maintaining normal levels of actin cable assembly and polarized cell growth (see figure).
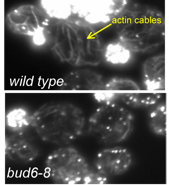 Earlier work from the Goode lab had shown that Bud6 enhances formin-mediated actin assembly in vitro (Moseley et al., 2004), but had left open the question of whether Bud6 stimulates the nucleation or elongation phase of filament growth (an important mechanistic distinction), and whether the activities of Bud6 are important in vivo. Graziano and collaborators dissected Bud6 mechanism by: (a) generating mutations in Bud6 that separately disrupt its interactions with formins (bu6-35) and actin monomers (bud6-8), (b) using TIRF (total internal reflection fluorescence) microscopy to visualize the effects of Bud6 and formins on individual actin filaments polymerizing in real time, and (c) performing a genetic analysis of bud6 alleles. They made three important observations. First, Bud6 enhances the nucleation rather than elongation phase of actin assembly, in sharp contrast to another formin ligand, profilin, that enhances elongation. Second, this activity of Bud6 requires its direct interactions with both the formin and actin monomers, suggesting that Bud6 recruits monomers to the formin to help assemble an actin ‘seed’. Third, genetic perturbation of these activities of Bud6 results in reduced levels of actin cable formation in vivo, in turn causing defects in polarized secretion and cell growth.
Earlier work from the Goode lab had shown that Bud6 enhances formin-mediated actin assembly in vitro (Moseley et al., 2004), but had left open the question of whether Bud6 stimulates the nucleation or elongation phase of filament growth (an important mechanistic distinction), and whether the activities of Bud6 are important in vivo. Graziano and collaborators dissected Bud6 mechanism by: (a) generating mutations in Bud6 that separately disrupt its interactions with formins (bu6-35) and actin monomers (bud6-8), (b) using TIRF (total internal reflection fluorescence) microscopy to visualize the effects of Bud6 and formins on individual actin filaments polymerizing in real time, and (c) performing a genetic analysis of bud6 alleles. They made three important observations. First, Bud6 enhances the nucleation rather than elongation phase of actin assembly, in sharp contrast to another formin ligand, profilin, that enhances elongation. Second, this activity of Bud6 requires its direct interactions with both the formin and actin monomers, suggesting that Bud6 recruits monomers to the formin to help assemble an actin ‘seed’. Third, genetic perturbation of these activities of Bud6 results in reduced levels of actin cable formation in vivo, in turn causing defects in polarized secretion and cell growth.
Until now, formins were thought to nucleate actin assembly by themselves, which is mechanistically distinct from the Arp2/3 complex (another major actin nucleator). Efficient nucleation by Arp2/3 requires the addition of a nucleation-promoting factor (NPF) such as WASp or WAVE, which recruits actin monomers. Graziano et al. reveal that some formins are similar to Arp2/3 in that they too require an NPF for robust nucleation. Their findings also uncover unanticipated mechanistic parallels between the two systems, since in each case nucleation requires both an actin filament end-capping component (formin or Arp2/3) and an actin monomer-recruiting factor (Bud6 or WASp).
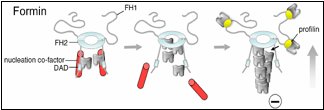 How well is this formin-NPF mechanism conserved? Clues to this question have recently emerged from other studies. A paper published last year in The Journal of Cell Biology by the Goode lab, working in collaboration with the labs of Niko Grigorieff (Brandeis) and Gregg Gundersen (Columbia), implicates the human tumor suppressor protein Adenomatous polyposis coli (APC) in functioning as a formin NPF (Okada et al., 2010). Another study published in The Proceedings of the National Academy of Sciences by the labs of Mike Eck (Dana Farber Cancer Institute), Margot Quinlan (UCLA), and Avital Rodal (Brandeis), suggests that Spire, which is conserved in mammals and flies, may serve as a formin NPF (Vizcarra et al., 2011). Bud6, Spire, and APC all bind multiple actin monomers and interact with the C-terminus of formins to enhance actin assembly, suggesting that they may have related mechanisms and perform functionally analogous roles.
How well is this formin-NPF mechanism conserved? Clues to this question have recently emerged from other studies. A paper published last year in The Journal of Cell Biology by the Goode lab, working in collaboration with the labs of Niko Grigorieff (Brandeis) and Gregg Gundersen (Columbia), implicates the human tumor suppressor protein Adenomatous polyposis coli (APC) in functioning as a formin NPF (Okada et al., 2010). Another study published in The Proceedings of the National Academy of Sciences by the labs of Mike Eck (Dana Farber Cancer Institute), Margot Quinlan (UCLA), and Avital Rodal (Brandeis), suggests that Spire, which is conserved in mammals and flies, may serve as a formin NPF (Vizcarra et al., 2011). Bud6, Spire, and APC all bind multiple actin monomers and interact with the C-terminus of formins to enhance actin assembly, suggesting that they may have related mechanisms and perform functionally analogous roles.
Although the requirement of NPFs increases the complexity of the formin mechanism, it offers an explanation for how cells simultaneously overcome two prominent barriers to actin assembly found in vivo – actin monomer binding proteins (e.g. profilin) that suppress formation of an actin nucleus and capping proteins that terminate growth by associating with the growing end of the filament. NPFs can facilitate nucleation by recruiting actin monomers in the presence of profilin, and formins protect growing ends of filaments from capping proteins. Future work will focus on identifying new formin-NFP pairs, defining the cellular processes with which they are associated, and distinguishing the underlying mechanistic differences among each set.
