Along with their collaborators in the Scott group at the University of Michigan, Tom Pochapsky’s group in the Chemistry Department recently published their findings in the Nature journal Chemistry Communications regarding a novel class of inhibitors for cytochrome P450 (CYP) enzymes. The human genome encodes fifty-seven different CYPs, and those enzymes are involved in a wide variety of biochemical processes, including steroid and prostaglandin biosynthesis, drug activation and metabolism, and detoxification of xenobiotics. Some CYPs, including CYP17A1 and aromatase, are targets for cancer treatments, with inhibitors of those enzymes being first line treatments for prostate and breast cancer, respectively. The problem with the currently used CYP inhibitors is their lack of specificity: they also inhibit other CYPs, including the liver CYPs responsible for drug clearance and detoxification. As such, those treatments can lead to drug sensitivities, and in extreme cases, liver failure.
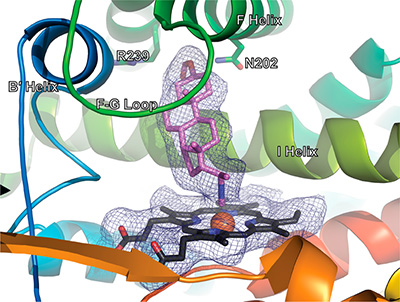
Their paper describes a new type of inhibitor that exhibits much higher selectivity for a particular CYP (CYP17A1 in this case), in that small differences in the structure of the molecule can make the difference as to whether the inhibitor binds to the target CYP, and if it does bind, whether it inhibits or not. Current work in both labs at U of M and Brandeis shows that the concept described in this paper can be extended to other CYPs, and patent applications have been submitted for this new class of inhibitor.
It is worth noting that an author on the paper (and co-inventor on one patent), Reethy Sundar ’21, was a Brandeis undergraduate, and that the second patent application has a Brandeis senior, Liam Flynn, as an inventor. Liam is still working in the Pochapsky lab, and will graduate next spring. This work was supported in part by the Brandeis I-Corps and Sprout programs, as well as the NIH.
Selective steroidogenic cytochrome P450 haem iron ligation by steroid-derived isonitriles. Alaina M. Richard, Nathan R. Wong, Kurt Harris, Reethy Sundar, Emily E. Scott & Thomas C. Pochapsky. Communications Chemistry. Volume 6, Article number: 183 (2023).
Thank you to Tom Pochapsky for this contribution.
