How does nature assemble nanoscale structures? Unlike the typical top-down methods for manufacturing, biological systems manufacture functional nanomaterials from the bottom up using a process called self-assembly. In self-assembly, individual ‘building blocks’ are encoded with instructions about how to interact with one another. As a result, ordered structures spontaneously form from a soup of building blocks through thermal fluctuations alone. Famous examples of self-assembling structures in nature include viral capsids, which protect the genetic material and orchestrate viral infections, and microtubules, which form part of the highway systems used for intracellular transportation. However, until recently, manufacturing similarly complex nanostructures from synthetic materials was out of reach because there were no methods for synthesizing building blocks with the kinds of complex geometries and interactions common to biological molecules.
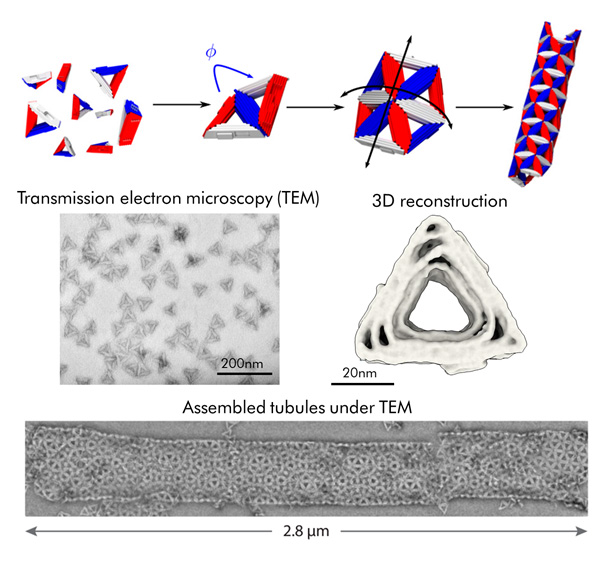
In collaboration with the Dietz Lab at the Technical University of Munich and the Grason Group at the University of Massachusetts Amherst, a team of scientists from the Rogers Lab, Hagan Group, and Fraden Lab in the Department of Physics at Brandeis developed a class of nanoscale particles that can overcome this hurdle. They designed and synthesized triangular building blocks using a technique known as DNA origami, in which the single-stranded DNA genome from a bacteriophage is ‘folded’ into a user-prescribed 3D shape using a cocktail of short DNA oligonucleotides. The triangular particles that they designed bind to other triangles through specific edge-edge interactions with bond angles that can be independently tuned to make a surface with programmable curvature.
Daichi Hayakawa, a Ph.D. student in the Rogers Lab, tuned the triangle design so that the particles would spontaneously assemble into a tubule with a programmed width and chirality. Interestingly, the assembled tubules were highly polymorphic. In other words, the width and chirality varied from tubule to tubule. Working together with the Hagan Group in Physics, the team rationalized this observation by considering the ‘softness’ of the edge interaction, which allows thermal fluctuations to steer assembly away from the target geometry. To constrain this polymorphism, the research team came up with an alternative method. By using more than one distinct triangle type to assemble a single tubule geometry, they found that they could eliminate some of these off-target structures, thereby making tubule assembly more specific.
In summary, this work highlights two avenues for increasing the fidelity of self-closing structures self-assembled from simple building blocks: control of the curvature through precise geometrical design and addressable complexity through increasing the number of unique species in the assembly mixture. Not only will this result be useful for constructing self-closing nanostructures through self-assembly, but it may also help us understand the role of symmetry and complexity in other self-closing structures found in nature.
Publication:
Geometrically programmed self-limited assembly of tubules using DNA origami colloids. Daichi Hayakawa, Thomas E. Videbaek, Douglas M. Hall and W. Benjamin Rogers. Proc Natl Acad Sci USA. 2022 Oct 25;119(43):e2207902119.


 Program Will Bestow Up to $100,000 to Promising Research Proposals
Program Will Bestow Up to $100,000 to Promising Research Proposals